Argon is compressed in a polytropic process with n 1.2 – Argon is compressed in a polytropic process with n = 1.2, a topic that unveils the intriguing relationship between pressure, volume, and temperature in thermodynamics. This process finds applications in various industries, and understanding its intricacies is crucial for engineers and scientists alike.
The polytropic process, characterized by the constant value of n, provides a valuable tool for analyzing gas compression and expansion. In this context, argon, an inert and abundant gas, serves as a suitable medium for studying polytropic compression due to its well-defined properties.
Polytropic Process
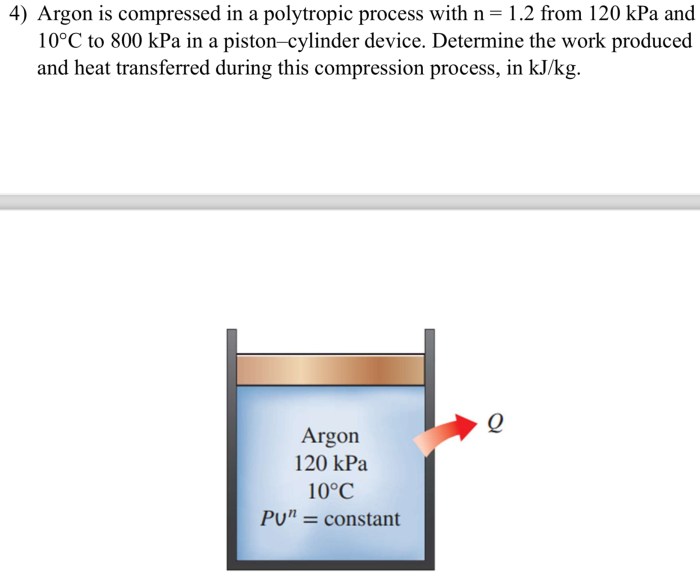
A polytropic process is a thermodynamic process in which the relationship between pressure, volume, and temperature can be expressed as P nV n-1= constant, where n is a constant.
Argon Gas
Argon is a noble gas with atomic number 18, atomic weight 39.948, and density of 1.784 g/L at room temperature. It is colorless, odorless, and non-toxic.
Argon is used in a variety of applications, including welding, lighting, and food preservation.
Argon is often used in polytropic processes because it is a stable gas that does not react with other gases.
Compression of Argon
Argon gas can be compressed using a compressor.
The factors that affect the compression of argon gas include pressure, temperature, and volume.
The following is a step-by-step procedure for compressing argon gas:
- Place the argon gas in a compressor.
- Close the inlet and outlet valves.
- Turn on the compressor.
- Monitor the pressure and temperature of the gas.
- Once the desired pressure is reached, turn off the compressor.
- Open the outlet valve to release the compressed gas.
n = 1.2

The value n = 1.2 is a specific case of a polytropic process.
When n = 1.2, the process is called an isentropic process.
An isentropic process is a process in which there is no heat transfer to or from the system.
Isentropic processes are often used in engineering applications because they are efficient.
Applications

The polytropic compression of argon with n = 1.2 is used in a variety of applications, including:
- Refrigeration
- Air conditioning
- Heat pumps
User Queries: Argon Is Compressed In A Polytropic Process With N 1.2
What is the significance of n = 1.2 in a polytropic process?
n = 1.2 represents a specific relationship between pressure and volume during compression or expansion, affecting the work done and heat transfer.
How does varying the value of n affect the compression process?
Varying n alters the path of the process on the pressure-volume diagram, influencing the efficiency and energy requirements.
What are the practical applications of argon compression with n = 1.2?
This process is employed in refrigeration systems, gas turbines, and other applications where controlled compression and expansion of gases are crucial.