Factors affecting the rate of chemical reactions worksheet – Embark on an enlightening journey into the captivating realm of chemical reactions with our meticulously crafted worksheet on factors affecting their rates. This comprehensive guide unveils the intricate mechanisms that govern the pace of these reactions, empowering you with a deeper comprehension of the chemical world.
As we delve into this exploration, we will uncover the profound influence of temperature, concentration, surface area, and catalysts on reaction rates. Real-life examples and thought-provoking experiments will illuminate the practical implications of these factors, fostering a holistic understanding of their significance.
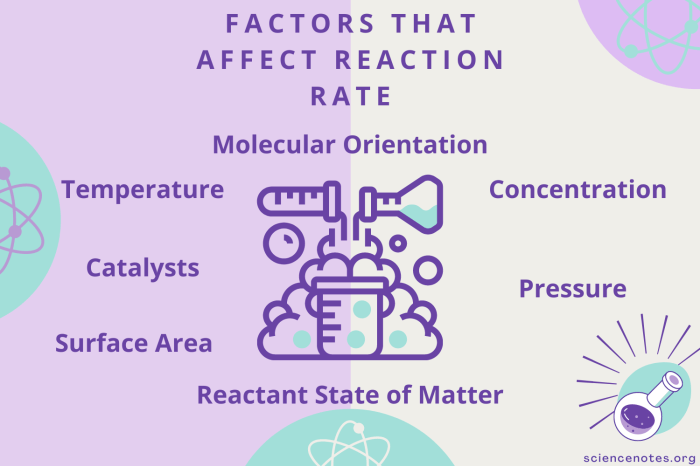
Factors Affecting Reaction Rates
Reaction rates measure the speed at which chemical reactions occur, influencing the efficiency and outcomes of various processes. Understanding these rates is crucial in fields like chemical engineering, medicine, and environmental science.
Temperature and Reaction Rates
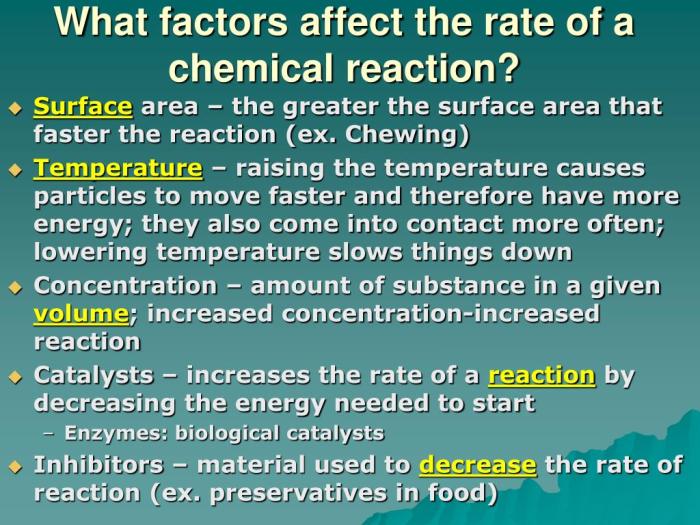
Temperature plays a significant role in reaction rates. As temperature increases, the average kinetic energy of molecules also increases, leading to more frequent and energetic collisions. This results in a higher probability of successful collisions and, consequently, a faster reaction rate.
The relationship between temperature and reaction rate is often described by the Arrhenius equation.
Concentration and Reaction Rates
The concentration of reactants directly affects the reaction rate. According to collision theory, the higher the concentration of reactants, the greater the number of collisions per unit time. This increased collision frequency leads to a higher reaction rate.
Surface Area and Reaction Rates
Surface area is a crucial factor in heterogeneous reactions, where reactants are in different phases (e.g., solid-liquid). Increasing the surface area of the solid reactant provides more active sites for collisions, enhancing the reaction rate.
Catalysts and Reaction Rates, Factors affecting the rate of chemical reactions worksheet
Catalysts are substances that increase the reaction rate without being consumed. They provide an alternative pathway with a lower activation energy, making the reaction proceed faster. Catalysts are widely used in industrial processes to enhance efficiency and selectivity.
Designing Experiments to Investigate Reaction Rates
Designing experiments to study reaction rates involves controlling and measuring the factors that influence them. Variables such as temperature, concentration, and surface area can be manipulated to observe their effects on the reaction rate. Techniques like spectrophotometry and gas chromatography can be used to monitor the progress of reactions.
Applications of Reaction Rate Studies
Understanding reaction rates has numerous applications. In chemical engineering, it helps optimize industrial processes by manipulating reaction conditions to achieve desired outcomes. In medicine, it aids in drug development and targeted therapies by controlling the rates of biochemical reactions. In environmental science, it contributes to understanding and mitigating pollution by studying the rates of environmental reactions.
General Inquiries: Factors Affecting The Rate Of Chemical Reactions Worksheet
What is the significance of reaction rates in chemical reactions?
Reaction rates play a pivotal role in determining the efficiency and practicality of chemical processes. Understanding reaction rates allows us to optimize reaction conditions, predict reaction outcomes, and design experiments effectively.
How does temperature influence reaction rates?
Temperature exerts a profound effect on reaction rates, as it affects the kinetic energy of reactants. Higher temperatures generally lead to faster reaction rates due to increased molecular collisions and activation energy.
What is the relationship between surface area and reaction rates?
Surface area plays a crucial role in heterogeneous reactions, where reactants are in different phases. Increased surface area provides more contact points between reactants, leading to a higher collision frequency and, consequently, faster reaction rates.